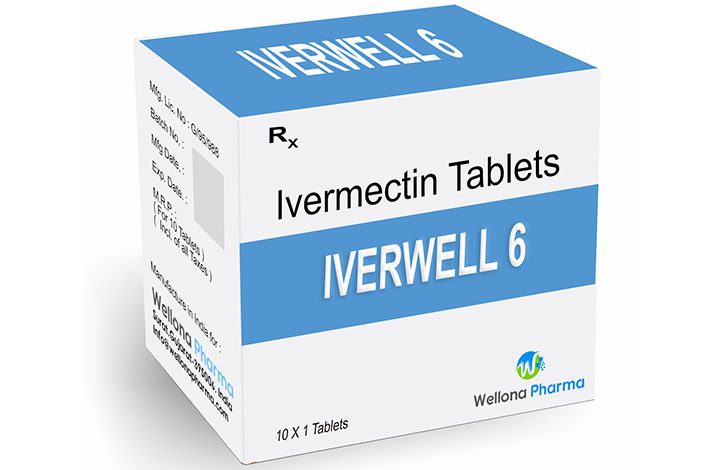
There is a flourishing formal and informal market for uncertified medicine which people are now using to treat Covid-19, including Ivermectin, an antiparasitic drug for treating worms in humans and animals.
The drug is under study as a possible agent against Covid-19 but scientists have warned that more evidence and data is needed before it is rolled out.
As a result, people have been encouraging each other in social media platforms to buy the drug as a means of protection against the fast spreading virus.
But this widespread use of the medicine is not recommended by public health physicians who advised it that the drug was not certified locally to be used against the new coronavirus.
In Zimbabwe, Ivermectin is currently registered for veterinary use only, with a few exceptions of external use on humans.
In a notice to the public, Zimbabwe College of Public Health Physicians (ZCPHP), warned people against its use.
“We have noted with concern the widespread use of the medicine ivermectin, and the flourishing formal and informal markets of ivermectin. Whilst we acknowledge the current crisis characterised by the rapid increase in the number of cases as well as tragic loss of lives, we should make sure any new treatments and approaches in responding to the pandemic remain in line with the evidence base and the robust tried and tested processes for regulating drugs and protecting public health in Zimbabwe,” said Dr Pamela Magande, ZCPHP president.
Dr Magande said people must wait for local certification systems to establish if the drug was safe before rushing to buy and self treat against Covid-19.
“Our country has a well-established, tried and tested regulatory process for bringing new treatments into clinical practice and it is important in the interest of public health for these systems and processes to be upheld,” she said and warned that any deviations from this robust process have both immediate and long-term implications for public health and medical practice.
The public health physician noted that “its license and safety status for Ivermectin was that it is currently only licensed for veterinary use in Zimbabwe.”
“It is NOT in our national treatment guidelines for Covid-19 or in the Essential Drugs List of Zimbabwe (EDLIZ). We have reviewed the current scientific evidence and international guidance on the use of Ivermectin for Covid-19 prophylaxis and treatment and our conclusion is that whilst there are a number of studies of differing quality and relevance to this issue, there is NO sufficient evidence to support its use,” Dr Magande emphasised.
Dr Magande appealed to the regulatory authorities such as the Ministry of Health and Child Care, Medical and Dental Practitioners Council, Medicines Control Authority of Zimbabwe, and Pharmacist Council of Zimbabwe to immediately act in the interest of public safety and stop forthwith the prescription and use of Ivermectin for the treatment and prevention of Covid-19.
“The ZPCHP continues to closely appraise emerging evidence on treatments for Covid-19 and will immediately advocate for recommendation for medicines that are found to be effective and safe. We recommend the urgent development (if not already in place) of a framework on research specifically targeted at developing and coordinating appropriate research for any new treatments and regimes purported to be preventative or curative for Covid-19,” she said.
This framework, according to Dr Magande could take advantage of existing arrangements, capacity and capability for research and development that exists across different agencies and sectors.
“This should also include cross border collaborations to ensure we learn from research being carried out in other countries, especially those with a similar context as Zimbabwe. We call upon medical practitioners to discharge their duties responsibly and in line with the code of conduct and professional expectations by not prescribing medicines that are not licensed for Covid-19,” she said.
Medical practitioners must not carry out small trials in their settings outside of the ‘robust, systematic processes’ that already exist and desist from bowing to public pressure or requests for none-licensed medicines, urged Dr Magande.
“They should also not further propagate information on the use of non-licensed medicines or treatment combinations that do not have an evidence base,” urged the public health physician,” she said.
Dr Magande also appealed to the public to comply with the proven public health measures for the prevention of Covid-19 transmission.
“Do not allow or demand the use of unregistered medicines such as ivermectin,” she said.