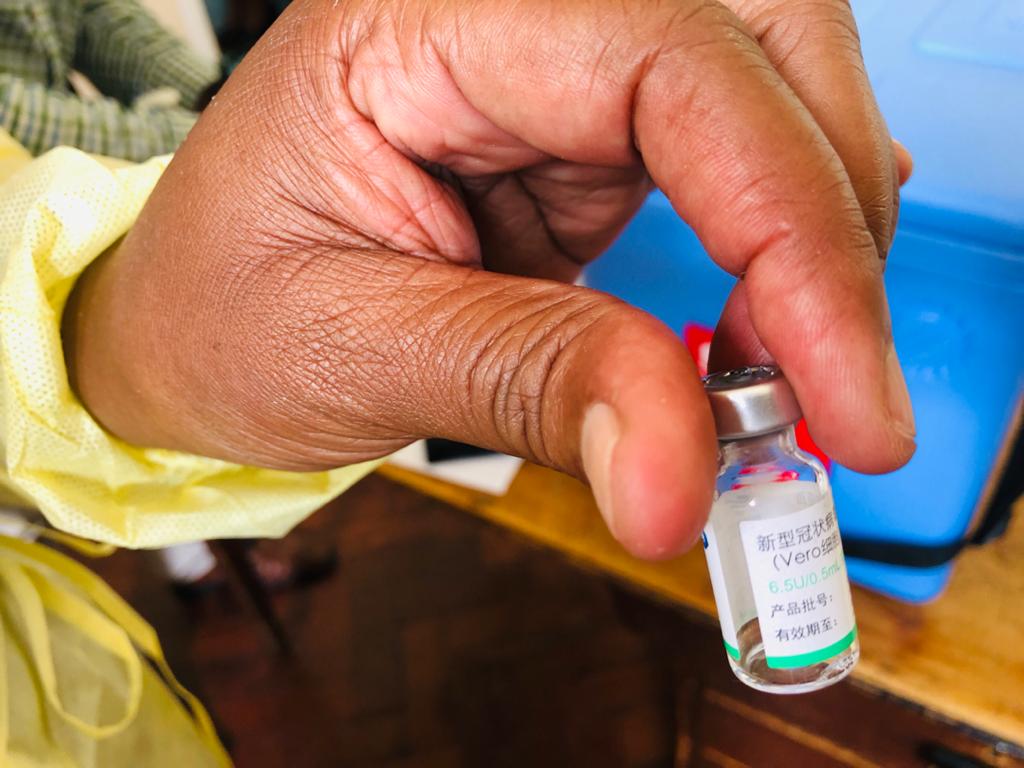
Zimbabwe has reached the half million mark for people who have been vaccinated against Covid-19, statistics from the Ministry of Health and Child Care show.
The country is aiming to vaccinate 10 million people, which is 60 percent of the population to achieve herd immunity needed to fight the novel coronavirus.
According to the health ministry’s daily reporting, 500 422 people received their first dose on May 7, 2021 while 140 340 have been fully vaccinated.
“22 248 received their first dose today bringing cumulative for first dose to 500 422 while 11 159 received their second dose bringing cumulative for second dose 140 340, as at 4pm.”
The Ministry of Health and Child Care.
Zimbabwe approved the use of four Covid-19 vaccines namely Sinopharm and Sinovac from China, Covaxin from India and Sputnik V from Russia.
The country has been mainly using Sinopharm and Sinovac in its vaccination campaign.

Zimbabwe also recorded five new local cases and one death on May 7, 2021 taking totakl number of Covid-19 cases to 38 403.
Out of the 38 403, 36 041 people have recovered and 1 576 have died from the novel coronavirus.
Meanwhile, China’s Sinopharm has been approved by the World Health Organisation (WHO) for “emergency use,” as announced by Director-General Tedros Adhanom Ghebreyesus Friday.
The director general said this approval would give countries “confidence to expedite their own regulatory approval.”
Sinopharm is the first of two jabs made in China under examination by the WHO to receive the go-ahead, meaning its safety, efficacy and quality has been checked. It can now be used by the international COVAX vaccine effort that supply vaccines to low and middle income countries.
Sinopharm also was given approval by the Strategic Advisory Group of Experts on Immunization (SAGE), which cleared it for use in those people who are “18 years and older in a two-dose schedule.”
The WHO approval is likely to accelerate the global roll out of vaccines, as reports said many governments around the world were waiting for the emergency use certificate to be granted before using a new medicine.
Sinopharm is also the first vaccine developed by a non-Western country to receive the WHO backing, although the vaccine had already been given to millions of people in China and elsewhere.
WHO had previously only approved Covid-19 vaccines made by Pfizer, AstraZeneca, Moderna, Johnson and Johnson.