The World Health Organisation (WHO) has recommended widespread use of the first-ever malaria vaccine to be deployed among children in sub-Saharan Africa and in other regions with moderate to high malaria transmission.
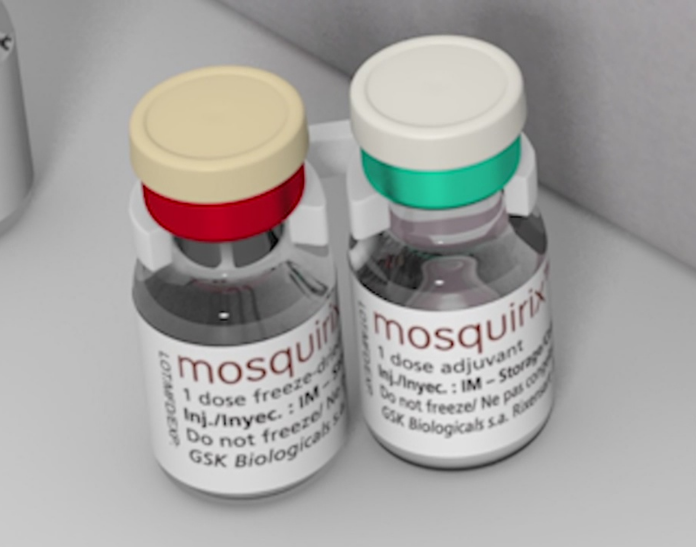
Called RTS,S/AS01 (RTS,S), also known as Mosquirix, the vaccine is based on results from an ongoing pilot programme in Ghana, Kenya and Malawi that has reached more than 800 000 children since 2019.
The malaria vaccine, RTS,S, acts against P. falciparum, the most deadly malaria parasite globally and the most prevalent in Africa.
Malaria remains a primary cause of childhood illness and death in sub-Saharan Africa and with more than 260 000 children in Africa under the age of five dying from the disease annually, this ‘groundbreaking’ vaccine, is expected to reduce child illness and deaths.
In announcing the development Wednesday, WHO Director-General Dr Tedros Adhanom Ghebreyesus, who started his career as a malaria researcher, said he longed for the day the world developed an effective vaccine “against this ancient and terrible disease.”
“This is a historic moment. The long-awaited malaria vaccine for children is a breakthrough for science, child health and malaria control,” he said in a briefing.
“Using this vaccine on top of existing tools to prevent malaria could save tens of thousands of young lives each year.”
In recent years, WHO and its partners had been reporting a stagnation in progress against the deadly disease and the Regional Director for Africa, Dr Matshidiso Moeti, noted that for centuries, malaria has stalked sub-Saharan Africa, causing immense personal suffering.
“We have long hoped for an effective malaria vaccine and now for the first time ever, we have such a vaccine recommended for widespread use. Today’s recommendation offers a glimmer of hope for the continent which shoulders the heaviest burden of the disease and we expect many more African children to be protected from malaria and grow into healthy adults,” she said.
Based on the advice of two WHO global advisory bodies, one for immunisation and the other for malaria, the health agency recommends the malaria vaccine should be provided in a schedule of four doses in children from five months of age for the reduction of malaria disease and burden.
Key findings of the pilots informed the recommendation based on data and insights generated from two years of vaccination in child health clinics in the three pilot countries, implemented under the leadership of the Ministries of Health of Ghana, Kenya and Malawi.
To date, more than 2.3 million doses of the vaccine have been administered in these three African countries, where WHO said the vaccine has a favourable safety profile.
“The vaccine introduction is feasible, improves health and saves lives, with good and equitable coverage of RTS,S seen through routine immunisation systems. This occurred even in the context of the Covid-19 pandemic.”
WHO noted the vaccine increased equity in access to malaria prevention, as data from the pilot programme showed that more than two-thirds of children in Ghana, Kenya and Malawi who are not sleeping under a bed net are benefitting from the RTS,S vaccine.
In areas where the vaccine has been introduced, there was no negative impact on the uptake of bed nets, other childhood vaccinations, or health seeking behaviour for febrile illness.
“There was significant reduction (30 percent) in deadly severe malaria, even when introduced in areas where insecticide-treated nets are widely used and there is good access to diagnosis and treatment while modelling estimates that the vaccine is cost-effective in areas of moderate to high malaria transmission,” said the health agency.
The next steps for the WHO-recommended malaria vaccine will include funding decisions from the global health community for broader rollout, and country decision-making on whether to adopt the vaccine as part of national malaria control strategies.
“Financing for the pilot programme has been mobilised through an unprecedented collaboration among three key global health funding bodies: Gavi, the Vaccine Alliance; the Global Fund to Fight AIDS, Tuberculosis and Malaria; and Unitaid,” WHO said.
The Malaria Vaccine Implementation Programme is coordinated by WHO and supported by in-country and international partners, including PATH (an international, nonprofit global health organisation), UNICEF and GlaxoSmithKline (GSK -the company behind the vaccine), which is donating up to 10 million doses of the vaccine for the pilot.
The RTS,S malaria vaccine is the result of 30 years of research and development by GSK and through a partnership with PATH, with support from a network of African research centres.
The Bill and Melinda Gates Foundation provided catalytic funding for late-stage development of RTS,S between 2001 and 2015.
Announcement on a malaria vaccine follows in the footsteps of huge vaccine advancements made around the Covid-19 pandemic.